
Pharma
Everything from one supplier
Weidmann Medical develops primary packaging for plastic containers, housings for liquid and solid medicines with safety components, drug delivery systems and other products for the pharmaceutical industry. We take care of the entire process – from part and tool design to packaging and supply chain management – all from a single source.
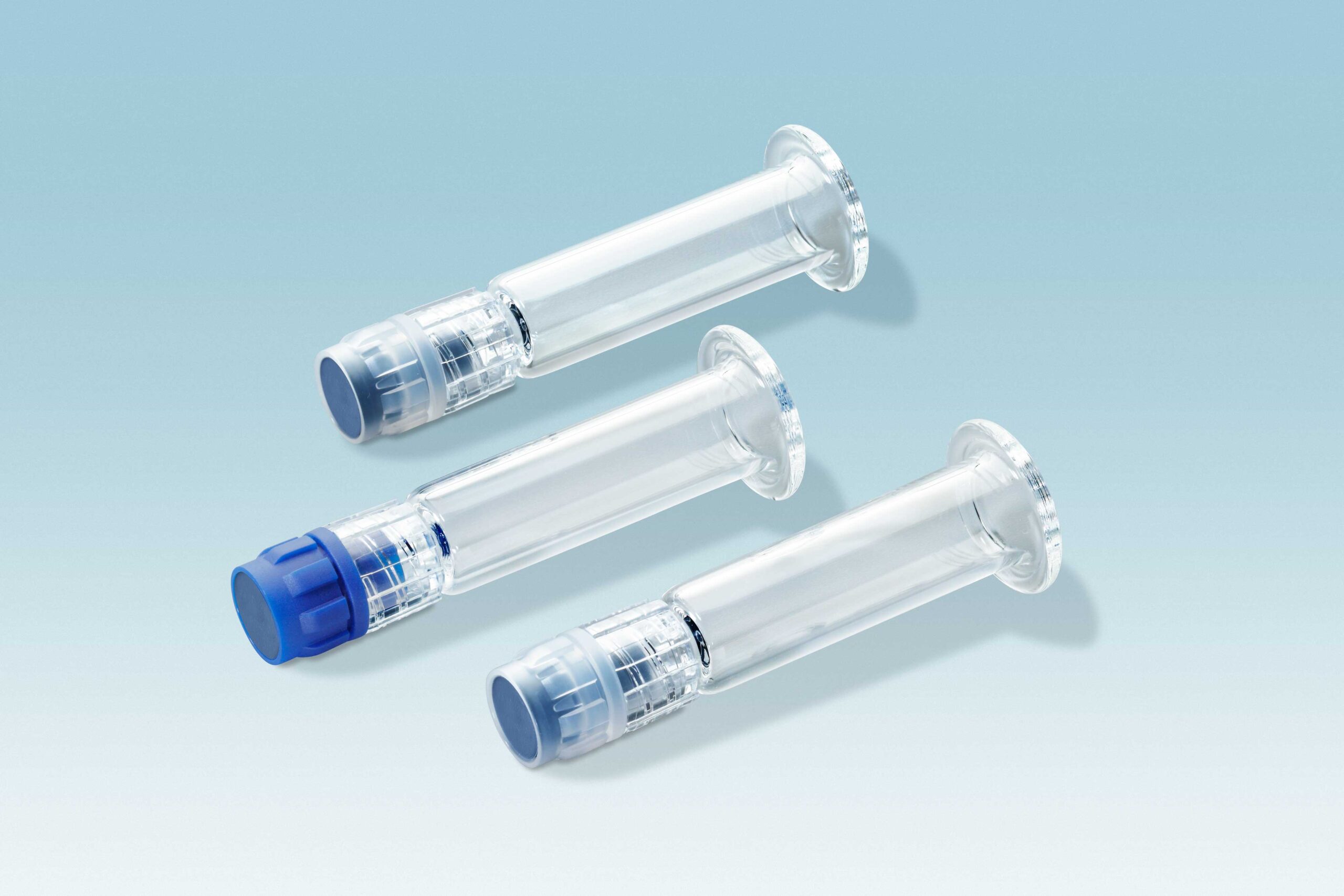
Luer lock closure for prefilled syringes
Fully automated assembly of syringe closures with tip cap

Needle shield
- Customized TPE solutions
- Mass production using highly sophisticated, multi-cavity molds
Our process
Product design
Industrialization
Process validation
End packaging and logistics
Weidmann Medical supports you in all steps of product development and, in parallel, creates the User Requirement Specification (URS), the Risk Management Documentation (FMEAs), and the Design History Files together with your team.
From a simple hand sketch through concept studies, product creation and design, rapid tooling and prototyping to raw material selection, cavity design, tool manufacture, and process development, we use the most modern technologies such as 3-D modeling via CAD-CAM, Moldflow Analysis, and static and dynamic calculations for part and tooling layouts.
After the User Requirement Specification (URS) is approved, Weidmann Medical develops, produces, and industrializes your product(s) according to cGMP guidelines. Our production technologies and control systems assure complete reproducibility of the entire production process.
All qualifications (DQ,IQ,OQ,PQ) and validation processes conform to ISO 13485 and have been certified by the DQS Agency since 2003. Weidmann Medical is audited by the FDA to the CFR820 standard and has been FDA-registered since 2013. All Cmk, Cpk-, Cp, and AQL requirements are fulfilled.
An ERP and supply chain management system enables an optimized logistics structure with both centralized and local production. Active controlling ensures continuous monitoring of the supply chain to guarantee quality and delivery reliability, while our supplier managers drive ongoing optimization.
